Sodium Benzoate Is Expected to Be More Soluble in an
Yes sodium benzoate will be MORE soluble in water because sodium benzoate is more polar Sodium Benzoate than benzoic acid. What affect is expected.

Solved Will Sodium Benzoate Be More Soluble In Water Than Chegg Com
052913 Abstract Sodium Benzoate is a common food preservative used in food products such as jams and jellies soft drinks pickles condiments etc and in tinned products in the market.

. Im pretty sure this is the right way to think about it but not 100 sure. Of the two would you predict to be more soluble in CHCI. Moreover it is soluble in non-polar solvents.
Sodium benzoate CAS No. Sodium benzoate is deemed safe and people generally dont exceed the ADI of 0227 mg per pound 05 mg per kg of body weight though some. No sodium benzoate will be LESS soluble in water because sodium benzoate is more polar than benzoic acid.
This is neutral quite non-polar. Is Glucose soluble in HCl NaOH and NaHCO3 because its soluble in water. Weve got the study and writing resources you need for.
When water is added to the ethanol solution it will dissolve the impurities leaving the pure hydrocarbon behind to recrystallize. Upon separating the lower layer and acidifying it with HCl benzoic acid is regenerated and because it has a low solubility in water it precipitates out. Therefore its IONIC bond breaks in water.
Sodium benzoate is not fat soluble which means that it does not concentrate in the body. Benzoic acid is separated from a known quantity of the sample by. Solution for the difference in the solubility of benzoic acid and sodium benzoate in water.
Sodium benzoate As in part B of exp. The melting point of benzoic acid solid is about 12241 C. Based upon this answer indicate whether the compound would be expected to be more soluble in water or more soluble in methylene chloride.
First week only 499. Therefore it is much more soluble in the non-polar diethyl ether than in water. Benzoic acid contains a polar region but is a relatively nonpolar substance.
Benzoic acid CAS No. It is more soluble in. According to my solubility table - sodium benzoate is soluble - 66 g in 100 mL water That is 660 g in 10 L water If we take Zobo drink to be equivalent to water then you can dissolve 660 g 20L 13200 g in 20 L water.
The product in the reaction scheme is sodium benzoate the salt of benzoic acid. The salt of this acid- sodium benzoate - doesnt have a partial charge to interact with the water via dipole dipole interactions instead it has a full positive and negative charge. Sodium benzoate is soluble in water so it can dissolve very well up to 60g per 100 g of the water depends on the temperature and it weakens the ionic bond of.
The previous reply would be correct but HCl is a stronger acid than benzoic acid so in HCl sodium benzoate would react to form benzoic acid which is insoluble in acidic aqueous solution. The Food and Drug Association FDA have recognised sodium benzoate as generally safe to consume in low doses. Sodium benzoate is the salt of benzoic acid.
So this is another difference between benzoic acid and sodium benzoate. However in acid solution benzoic acid is formed. Benzoic acid is used as an intermediate in the synthesis of different compounds primarily phenol 50 of the amount produced worldwide and caprolactam.
Sodium benzoate is more soluble in water than benzoic acid This is primarily due to the fact that sodium benzoate is O A a carboxylic acid B aromatic C ipophilic D D ionic E hydrophobic tnnther estion will save this response. Moreover benzoic acid is poorly water-soluble at room temperature but if we heat the compound it becomes more water-soluble. The denser lower water layer will thus contain the sodium benzoate.
Hydrocarbon is more soluble in ethanol which means the impurities are more soluble in water. However sodium benzoate is water-soluble at room temperature. Synthia Gratia Date of Submission.
Will sodium benzoate be more soluble in water than benzoic acid a. Determine whether each of the five compounds is predominately ionically or covalently bonded. Benzoic acid will be more soluble in water compared to 9-fluorenone because benzoic acid has an O-H group attached to it making it more polar.
The main antimicrobial effect of benzoic acid is due to the undissociated acid used for high acid foods with a pH lower than 45 eg soft drinks ciders and dressings but also applied in fruit salads jams jellies sauerkraut and margarine Søltoft. Sodium benzoate is 200 times more soluble in water. 532-32-1 is about 200 times more soluble in water.
You are right thats what gabriels-horn was hinting at. Sodium benzoate is formed from benzoic acid by what common type of chemical reaction. - I put soluble for all because it dissolves in water.
Effect of pH on sodium benzoate a food preservative Sheikh M Zakaria Person no. Benzoic is soluble in a solution of NaOH because the base forms the sodium salt with the acid to form sodium benzoate. The benzoate anion is not soluble in non-polar solvents because of its negative charge.
This means the ions are able to form even stronger dipole-ion interactions with. Is p-toluidine soluble in HCl NaOH and NaHCO3. Some doctors maintain that due to the small amount of sodium benzoate we are consuming it does not pose a definite risk to our health.
1 when the graphs are made properly the density of the liquid can be obtained via. Start your trial now. Although benzoic acid is more soluble in ether than in water the salt of benzoic acid being ionic and very polar is more soluble in water than in ether.
Naphthalene benzoic acid sodium benzoate ethyl 4-aminobenzoate ethyl 4-aminobenzoate hydrochloride. The amount of benzoic acid produced will not be significantly different Which is a more stable conjugate base. Sodium benzoate as flavor enhancer While used mainly as a food preservative sodium benzoate also acts as a flavor enhancer to soft drinks.
The solubility of benzoic acid is very low in water and thus the more soluble form sodium benzoate is commonly used Berk 2018. 65-85-0 is a white solid that is slightly soluble in water.

Solved Will Sodium Benzoate Be More Soluble In Water Than Chegg Com
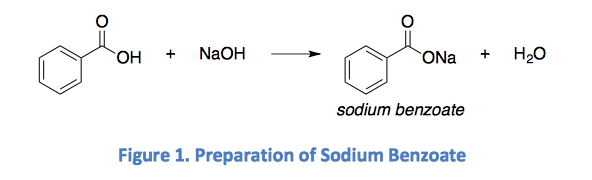
Solved 2 Figure 1 Shows The Conversion Of Benzoic Acid To Chegg Com
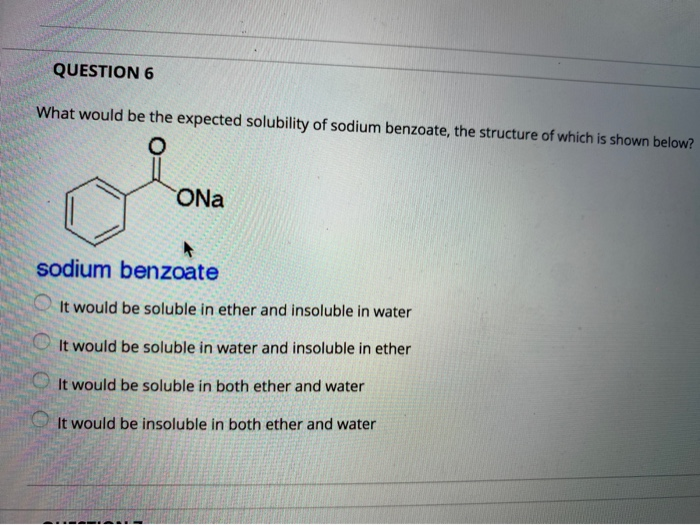
Solved Question 6 What Would Be The Expected Solubility Of Chegg Com
0 Response to "Sodium Benzoate Is Expected to Be More Soluble in an"
Post a Comment